
|
|
 Harry Morgan
Harry Morgan
DPhil student
Physical and
Theoretical Chemistry Laboratory
University of Oxford
South Parks Road
Oxford, OX1 3QZ
United Kingdom
E-mail: harry.morgan@chem.ox.ac.uk
ORCID: 0000-0001-9647-8807
MChem, University of Oxford
MSc, University of Oxford
Research interests:
- Transition Metal and Solid State Chemistry
- Mechanisms in single-atom catalysis
- Structural and electronic processes in perovskites
- Bonding in encapsulated clusters
Publications:
Improving Hydride Conductivity in Layered Perovskites via Crystal Engineering, H. W. T. Morgan, H. J. Stroud, and N. L. Allan, ChemRxiv, (2020), 10.26434/chemrxiv.12730553.v1
[Cu4@E18]4- (E = Sn, Pb): Fused Derivatives of Endohedral Stannaspherene and Plumbaspherene, L. Qiao, C. Zhang, C.-C. Shu, H. W. T. Morgan, J. E. McGrady, and Z.-M. Sun, Journal of the American Chemical Society, 142 (2020) 13288-13293, 10.1021/jacs.0c04815
A family of lead clusters with precious metal cores, C.-C. Shu, H. W. T. Morgan, L. Qiao, Z.-M. Sun and J. E. McGrady, Nature Communications, 11 (2020) 3477, 10.1038/s41467-020-17187-4
Pressure-induced transitions in 1-dimensional vanadium oxyhydrides Sr2VO3H and Sr3V2O5H2 and comparison to 2-dimensional SrVO2H, T. Yamamoto, H. W. T. Morgan, D. Zeng, T. Kawakami, M. Amano Patino, M. A. Hayward, H. Kageyama, J. E. McGrady, Inorganic Chemistry, 59 (2019) 15393, 10.1021/acs.inorgchem.9b02459
Structural isomerism in the [(Ni@Sn9)In(Ni@Sn9)]5- Zintl ion, C. Zhang, H. W. T. Morgan, Z.-C. Wang, C. Liu, Z.-M. Sun, and J. E. McGrady, Dalton Transactions, 48 (2019) 15888, 10.1039/C9DT03008E
Sr2FeIrO4: Square-Planar Ir(II) in an Extended Oxide, J. E. Page, H. W. T. Morgan, D. Zeng, P. Manuel, J. E. McGrady and M. A. Hayward, Inorganic Chemistry, 57 (2018) 13577, 10.1021/acs.inorgchem.8b02198
Ba6Nb4RuO18 and LaBa4Nb3RuO15 The structural consequences of substituting paramagnetic cations into AnBn-1O3 cation-deficient perovskite oxides, E. E. Kamil, H. W. T. Morgan, M. A. Hayward, Journal of Solid State Chemistry, 238 (2016) 267 , 10.1016/j.jssc.2016.03.040
Current projects:
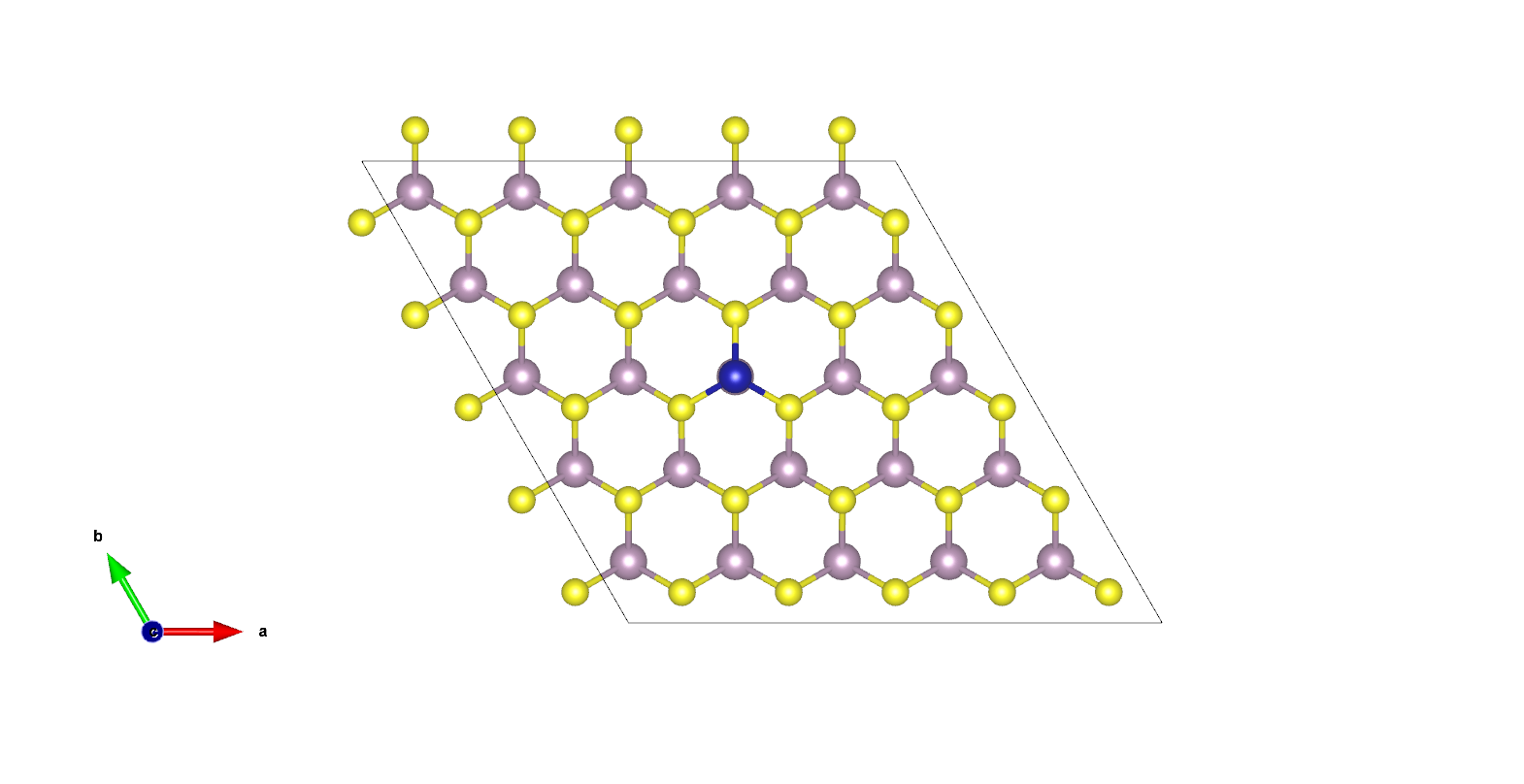 |
Hydrogen has the potential to play a starring role in the sustainable fuel economy of the future. For this to be practical, we need an efficient means of producing hydrogen. In collaboration with the Tsang group (Oxford) we are studying the ability of single transition metal atoms adsorbed onto MoS2 monolayers to catalyse the conversion of water to hydrogen (the “hydrogen evolution reaction”, or HER). A single-atom active site incorporated into a two-dimensional periodic system puts this catalyst on the frontier of the traditional classes of homogeneous and heterogeneous and is therefore a challenge to experimental and computational study. By exploring its dual nature with a multifaceted computational approach we aim to understand the electronic details of the catalyst and aid the design of next-generation sustainable technology.
We are exploring another aspect of hydrogen technology in
the emerging field of oxyhydride chemistry. These
solid materials are of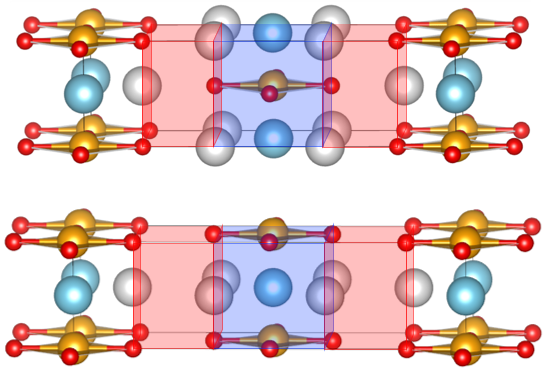 interest both for the unusual bonding properties of the
hydride anion and for the low-dimensional structures which
they adopt, derived from perovskite parents.
Pressure-induced structural and electronic transitions in
SrVO2H and Sr2VO3H
are governed by the sigma symmetry of the hydrides and the
incompressibility of the V-O bonds, and hydride conduction
mechanisms in compounds like Ba2ScO3H
are determined by local flexibility in different regions of
the Ruddlesden-Popper framework. These examples show the
ways in which the properties of transition metal oxyhydrides
are dominated by the dimensionalities of their structures.
interest both for the unusual bonding properties of the
hydride anion and for the low-dimensional structures which
they adopt, derived from perovskite parents.
Pressure-induced structural and electronic transitions in
SrVO2H and Sr2VO3H
are governed by the sigma symmetry of the hydrides and the
incompressibility of the V-O bonds, and hydride conduction
mechanisms in compounds like Ba2ScO3H
are determined by local flexibility in different regions of
the Ruddlesden-Popper framework. These examples show the
ways in which the properties of transition metal oxyhydrides
are dominated by the dimensionalities of their structures.
Ligand-free Zintl ions have held the attention of chemists
for their high-symmetry geometries which continue to
challenge our understanding of chemical bonding. Synthetic
advances have brought clusters containing multiple
encapsulated transition metal atoms, opening up the
possibility of metal-metal bonds within the clusters. We
have recently explored the balance of covalent and
electrostatic interactions in stabilizing clusters in which
lead encapsulates copper,
silver
and gold.